Abstract
Chimeric antigen receptor (CAR) T-cells are genetically engineered T-cells with potent biocidal activity against respective target-expressing cells. Recently, CAR T-cells have been successfully used clinically to eradicate B cell-derived malignancies by targeting B-cell lineage specific surface antigens (e.g. CD19, BCMA). However, several limitations of current single-antigen targeting CAR T-cell therapies are becoming evident: a) low target tumor-antigen expression might lead to low CAR T-cell targeting efficacy; b) single antigen-targeting might lead to rapid selection of tumor cells with low or loss of antigen expression; c) single antigen-targeting does frequently not generate a high tumor-selectivity as tumor-antigens are frequently also expressed on healthy tissues; d) production of single-antigen CAR T-cells is time- and resource-consuming but results in effectors that target only one antigen; e) on-target off-tumor as well as off-target side-effects are difficult to control without terminally eliminating CAR T-cells in the recipient. While a-c) might be overcome by combinatorial tumor-antigen targeting, d-e) might be addressed by production of Universal CAR T-cells that recognize a specific tag on selective bridging molecules with short half-life.
To address some of these limitations in principle, we have here developed universal CAR T-cells targeting fluorescein, as well as fluorescein-labeled antibody-constructs directed against several cell surface antigens, that would serve as versatile, combinatorial selective linkers and, upon target binding, also CAR T-cells activators. We then tested the system on human acute myeloid leukemia (AML) cell lines and primary patient AML blasts. Specifically, we engineered CAR T-cells, termed FluA-CAR T-cells, to display the anti-fluorescein engineered lipocalin FluA, which mediated recognition of randomly or site-specifically fluorescein-labeled antibodies in IgG or short half-life diabody (Db) format, directed against the frequently AML expressed antigens CD33, CD117 and CD371. Site-specific chemical modification methods and cysteine-tagged Db mediated the strongest AML killing results in vitro over a broad range of antibody concentrations.
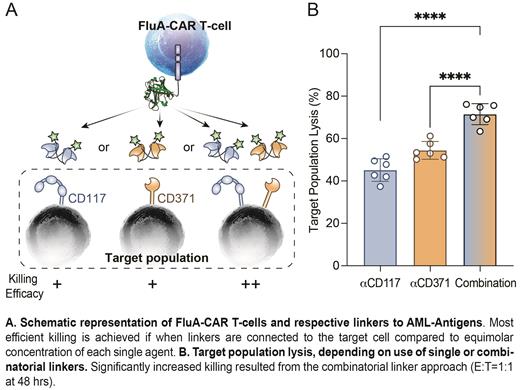
We then hypothesized that FluA-CAR T-cells, targeting AML cells via combinatorial use of linker molecules adapted to specific AML antigen-expression profiles, would allow to most efficiently eliminate AML cells in a dose- and timely-regulated fashion. To this end, we tested single and combinatorial use of fluoresceinated anti-CD117 Db and anti-CD371 Db on MOLM13 AML cell lines, engineered to be either CD117 highCD371 neg, CD117 negCD371 high, or CD117 highCD371 high. Indeed, combination of anti-CD117 and anti-CD371 Db-linkers, resulted in significantly improved MOLM13 cell lysis compared to equimolar concentrations of single agents in vitro (example figure). We then tested the same approach, targeting CD117 +CD371 + primary AML cells from two different patients, using either patient-derived or allogeneic FluA CAR T-cells. Again, the combinatorial use of linkers generated a significantly higher AML cell lysis than the use of single Db linkers.
We thus here provide proof-of-concept for the generation of highly potent universal targeting FluA CAR T-cells from healthy donors and AML patients. By choosing suitable CAR-adaptors with respect to their conjugation chemistry and size, it is possible to tightly regulate CAR-T cell activity against CD33, CD117 and CD371 expressing AML cells and likely any tumor cell expressed antigen. Short half-life small molecule linkers will allow to control FluA CAR T-cell on-off activity and combinatorial use of linkers will allow to maximize anti-tumor activity and to minimize on-target off-tumor toxicity.
Myburgh: University of Zurich: Patents & Royalties: CD117xCD3 TEA. Neri: Philogen S.p.A.: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Multiple patents on vascular targeting; ETH Zurich: Patents & Royalties: CD117xCD3 TEA. Manz: CDR-Life Inc: Consultancy, Current holder of stock options in a privately-held company; University of Zurich: Patents & Royalties: CD117xCD3 TEA.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal